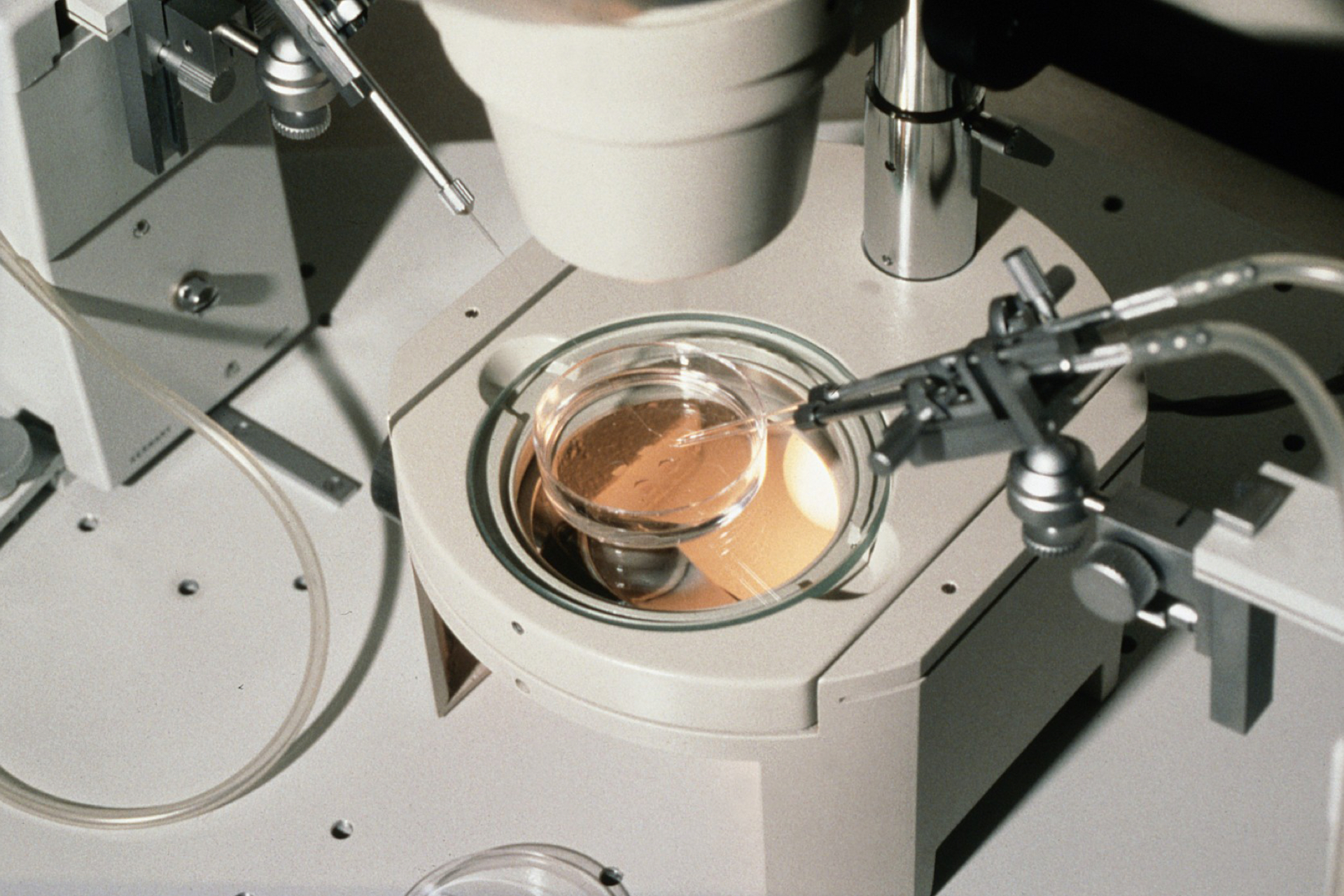
Australia saw the birth of its first baby conceived via capacitation in-vitro maturation or CAPA-IVM treatment last week.
CAPA-IVM differs from IVF in that eggs are retrieved from the woman earlier in the menstrual cycle and then matured in cell culture. This procedure reduces the length of hormone treatment for the woman from two weeks to approximately two days. The technique is relatively new. Births following its use were first reported in February 2020, since when approximately 150 babies have been born worldwide. Due to the lower amount of drugs used, it is also expected to be significantly cheaper than IVF. Experts disagree about whether this will reduce the likelihood of ovarian hyperstimulation syndrome.
'The burden is much less on the patient,' said Professor Robert Gilchrist, director of research in the School of Women's & Children's Health at UNSW Sydney, and co-developer of the treatment. 'We've got quite a special treatment here because these women have only two days of hormones and have a reasonable prospect of getting pregnant.'
CAPA-IVM is furthermore a development beyond standard IVM. Once the egg is removed from the body in standard IVM, it matures very quickly, which can lead to unhealthy eggs and a lower chance of pregnancy. The CAPA process delays maturation by around 24 hours, causing the egg to mature more 'healthily'.
'Many years of blood, sweat and tears have gone into this. Countless hours of research over two decades, so this is a very proud moment,' Professor Gilchrist continued. 'To be able to take a discovery from the lab into a clinic and make a real difference... is very special.'
However, the novelty of the technique means that there is still a lot we do not know about it.
'Like all forms of IVF, in which oocytes and embryos are maintained in the laboratory outside the body, the artificial environment may also impose risks,' Professor Daniel Brison, a clinical embryologist from the University of Manchester told MailOnline.
While licensed clinics can perform CAPA-IVM in the UK, the Human Fertilisation and Embryology Authority (HFEA) currently cautions against its widespread use in the UK.
'This means we can't be 100 percent confident of its safety until there have been more healthy births and researchers have been able to observe the development of children as they've grown up,' Rachel Cutting from the HFEA told MailOnline.



Leave a Reply
You must be logged in to post a comment.