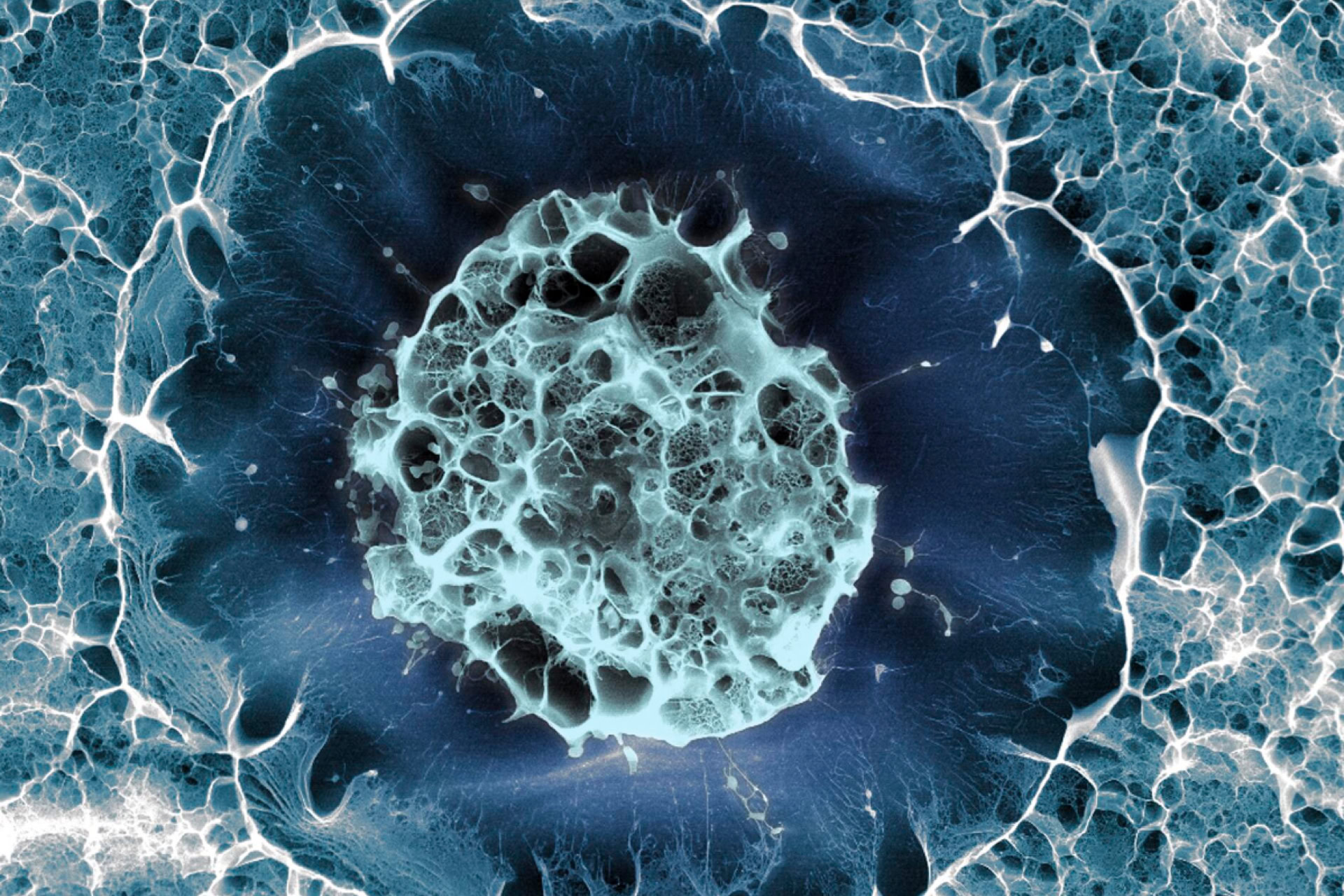
The University of Minnesota, US, has applied to the Food and Drug Administration (FDA) for permission to begin clinical stem cell trials in humans. If the FDA approves the trials, the University would become the first public research institute in the country to begin work looking at the therapeutic use of embryonic stem cells (ES) cells) in humans. John Wagner, the clinical research director at the University's stem cell Institute told state lawmakers that one set of trials will use stem cells derived from adults, while another set will use ES cells. Wagner said the University was currently briefing the FDA, National Institutes of Health and the United Nations. 'This is a tremendous undertaking for a tremendous gain', he said, but refused to comment further.
At the same time, legislation both for and against ES cell research is currently being debated by the Minnesota Senate and House of Representatives. Two weeks ago, a Minnesota Senate Health and Family Security Committee approved a bill that would allow regulated ES cell research in the state, as well as research on embryos left over from fertility treatments. The bill also eliminates any doubt as to whether the ES cell research programme at the state university - funded by private donations - is legal. Because it is privately funded, it is outside the limitations placed on US stem cell researchers by President Bush in August 2001. Researchers at the university, headed by Dr Catherine Verfaillie, want to be able to work on both adult and ES stem cells, to determine which the best are scientifically. But, after the university announced the expansion of its work to ES cells, some lawmakers introduced competing legislation to prevent such research taking place, although they later said that a 1973 state law already outlaws it. Similar 'companion' bills have been introduced to the state House.
Meanwhile, the Massachusetts Joint Committee on Health Care has approved a bill that would support embryo stem cell research in the state, while outlawing human reproductive cloning. Local companies and universities involved in stem cell research are given support by the bill, which is similar to those already passed in California and New Jersey. Robert Lanza, of Massachusetts biotechnology company Advanced Cell Technology, said that the bill would strengthen plans by Boston's Harvard University to establish a new stem cell research centre. He added 'this is an exciting time in the stem cell field and we'd really like to see Massachusetts step up to the plate. Why should we leave it to other states to make a statement?', he said.
The state of Delaware, however, has decided to delay the progression of a similar bill, which would ban human reproductive cloning but allow cloning for medical research (therapeutic cloning). The House Health and Human Development Committee will now take no action on the bill until supporters and opponents have presented their arguments to the full House. According to reports, committee members have 'struggled with the scientific and social consequences of the bill', and the testimonies are required in order to 'let House members hear the issues surrounding the controversy before the committee acts on recommending the bill'.
Sources and References
-
Lawmakers endorse stem cell research bill
-
Stem Cell Research Bill Advances
-
Delaware House Committee Delays Action on Cloning Bill, Requests Testimony From Bill Supporters, Opponents
-
University Plans Human Stem Cell Trials



Leave a Reply
You must be logged in to post a comment.