Any healthcare professional undertaking clinical procedures in fertility clinics must be appropriately trained to ensure competency in their technique. For lab staff, this can only be achieved when gametes and embryos surplus to treatment requirement, are available for training purposes. These can only be acquired when patients attending fertility clinics have provided appropriate consent by completing consent forms.
Recent years have seen a shift in completion of consent forms to online formats across the UK. Historically, patients would complete paper consent forms in clinics after a consultation with a doctor or nurse. Now, online consent forms are introduced and explained to patients during initial consultations in clinics, and then patients complete the online information modules and consent forms at home.
The Human Fertilisation and Embryology Authority (HFEA) code of practice states that electronic consent formats should not substitute a face-to-face, video or phone conversation between patients and/or their partner and clinic staff about consent, treatment options and their implications.
Completion of consent forms online is beneficial for patients and clinics for several reasons. For patients, it allows the completion of consent forms in their own time, and space. It also reduces the need for extended appointments to complete paper consent forms, limiting the time patients must spend in the clinic. For clinics, it allows the standardisation of delivery of information to patients, creating an efficient platform for sharing large volumes of information.
Online consent forms also reduce the length of appointments, reducing patient contact time for staff and easy amendment and/or addition of consent forms if required. Electronic platforms hypothetically improve security, as it minimises the risks of confidentiality breaches as well as storage capacity within clinics. Lastly, it contributes to improving sustainability for clinics, adopting into a paperless system approach. While there are several promising attributes to the transition between paper and online consent forms, there are concerns that fewer patients are consenting to the use of their gametes and embryos for training purposes since the implementation of online consent forms.
To explore this, we investigated consent forms completed by 100 patients who completed paper consent forms before the implementation of online consent forms in the Assisted Conception Unit, Ninewells Hospital, Dundee, in June 2021, and 100 online consent forms completed afterwards. Fifty percent of forms were completed by men and fifty percent were completed by women. (A graphic representation of our findings is provided at the end of this article.)
We found that 71 percent of female patients consented to their gametes being used for training, and 63 percent for their embryos; 70 percent of male patients consented to their gametes being used for training, and 64 percent for their embryos, when completing paper consent forms.
When completing consent forms online, 57 percent of female patients consented to their gametes being used for training, and 49 percent to use of their embryos; 59 percent of male patients consented to their gametes being used for training, and 49 percent to use of their embryos. There was a significant decrease in the number of patients, both male and female, consenting to their gametes and embryos being used for training purposes following conversion to online consent forms. This raises the question whether completing consent forms online has affected patient understanding. Improving patient awareness and understanding is critical to prevent further decline in availability of gametes and embryos for training.
On 1 July 2022, UK legislation changed to allow the extension of storage consent for patients with cryopreserved gametes and embryos beyond ten years (see BioNews 1111). This involved updating the HFEA's consent forms to reflect this change, and with this was included an expansion detail regarding what consenting to gametes and embryos being used in training involves. Importantly, it states that any gametes or embryos being used for training purposes will not affect patients' treatment in any way.
Another interesting addition was the opportunity for patients to consent to the storage of gametes and embryos specifically for training purposes, with specific storage period options. We decided to investigate whether this change had made patients more likely to consent to their gametes and embryos being used for training of staff.
A separate cohort of patients who had completed the updated consent forms implemented in July 2022 was analysed next. When completing updated consent forms online, 62 percent of female patients consented to their gametes being used for training, and 52 percent to their embryos being used for training; 65 percent of male patients consented to their gametes being used for training, and 56 percent to their embryos being used for training. No significant change was found with the new forms.
The outcomes of this audit sparked concern. Reduced availability of gametes and embryos for training purposes may negatively impact training opportunities and delay development of staff. However, the updated consent forms offer fertility clinic staff and clinics an opportunity to create a bank of gametes and embryos stored for training purposes. This may prove beneficial for staff as training can be pre-planned and flexible to fit around clinical workload.
Completing consent forms can be an overwhelming process for patients. It is essential that we provide patients with the correct and necessary information to allow informed consent, and in the most appropriate format for patients to understand key details before completing consent forms. We cannot deny that the use of online consent forms offers huge advantages for clinics, staff and patients, but we must be cautious not to overwhelm patients and optimise the use of online platforms for staff and patients.
We must ensure electronic platforms do not replace the benefits patients receive when completing paper consent forms in clinics, and monitor progress to measure their success, for example by quantifying time saved for healthcare professionals and collecting patient feedback. Perhaps, as with many aspects of life in a post-pandemic era, the best approach is to adopt a hybrid system for completing consent forms. To maximise understanding for patients and, crucially, assure informed consent, clinics may look to offering additional options, such as: in-person or virtual information sessions, drop-in sessions for patients to attend, listen and ask questions.
Below: Proportion of patients consenting to use of gametes (left) and embryos (right) for training purposes in the HFEA woman's consent to treatment (WT, top) and HFEA men's consent to treatment (MT, bottom) forms using online and paper consent platforms.
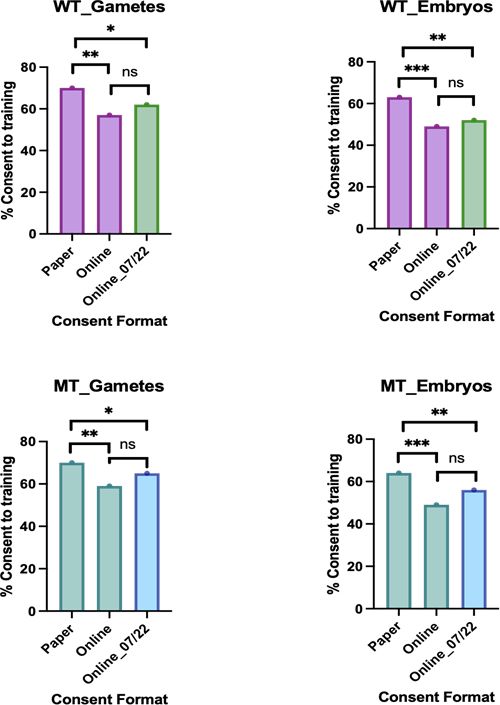
Note: Purple bars represent completion of WT version 9 and WT green bars represent completion of WT version 11. MT teal bars represent completion of MT version 7 and MT blue bars represent completion of MT version 8.






Leave a Reply
You must be logged in to post a comment.