Add-ons at the time of IVF have become commonplace, despite a general lack of evidence that they are effective and safe. Some add-ons are tracked by the Canadian CARTR Plus register. The most recent data extracted from that register confirm the continuing upward trend in the use of add-ons.
By 2020, 74 percent of all fresh non-preimplantation genetic testing for aneuploidy (PGT-A) cycles used intracytoplasmic sperm injection (ICSI); 41 percent used assisted hatching on fresh embryos; 29 percent of cycles were committed to PGT-A and 6.4 percent of cycles consented to participate in an endometrial receptor assay.
In Canada, the regulation of clinical services falls on the ten provincial and three territorial governments. The federal government's authority over the pharmaceutical, scientific and laboratory components of fertility services is divided and 'siloed' among several branches of Health Canada. None of them has fertility services listed as a priority.
In response to concerns about the marketing of add-ons, the European and British fertility services sectors have agreed that add-ons without strong evidence of their safety and/or effectiveness should only be offered in a research setting. In the UK, the Human Fertilisation and Embryology Authority (HFEA) has worked with health professionals, scientists, and regulatory authorities to develop an updated add-ons rating system (see BioNews 1226). Regulatory advice is backed by consumer protection laws. The European Society of Human Reproduction and Embryology (ESHRE) has recently published similar good practice recommendations on add-ons in reproductive medicine.
Using a Delphi methodology supported by Choosing Wisely Canada, a working group of Canadian Fertility and Andrology Society (CFAS) members created a list of the top five unnecessary and costly diagnostic and therapeutic fertility interventions. The guidance document was approved in 1999 and subsequently published. It was anticipated that this list would assist patients who were brave enough to question providers of fertility services when these add-ons were recommended to them. The recommendations have largely been ignored by providers, patient support organisations and governments. This modest list of unnecessary add-ons included PGT-A; laser-assisted hatching of fresh embryos; the use of immunological medications to improve pregnancy rates; sperm DNA fragmentation testing and high-dose gonadotropins for ovarian stimulation. Not addressed by the CFAS were ICSI for non-severe male factor infertility or in PGT-A, endometrial receptivity assays or time-lapse imaging and incubation.
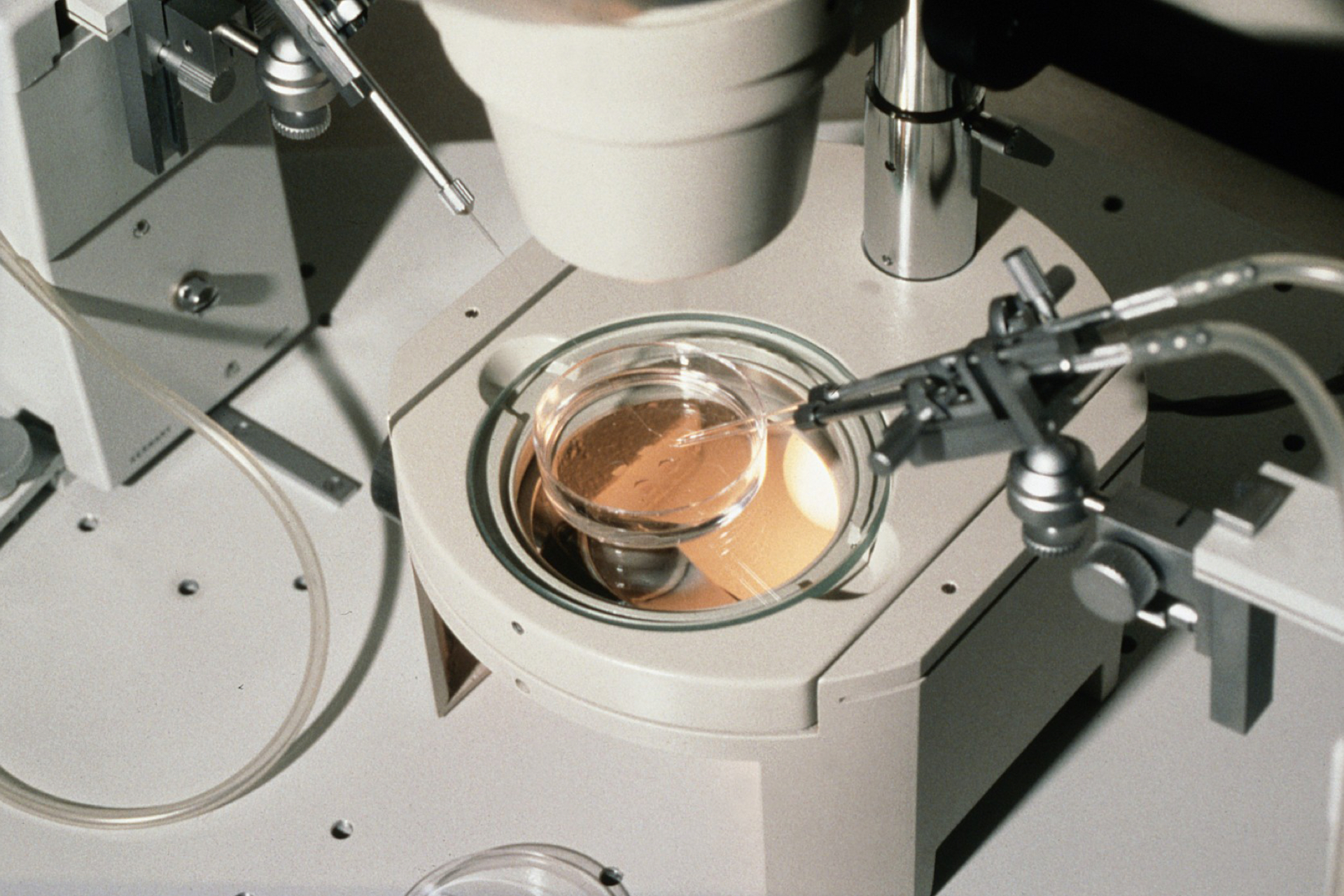
One particular add-on that was used in Canada, was the Augment technique – marketed by US company OvaScience. Introduced as a treatment for low-quality IVF embryos, this technique required a woman to undergo a laparoscopic ovarian biopsy under general anaesthesia. Mitochondria were extracted from the egg precursor cells in the biopsy. These mitochondria were then injected into the patient's egg, along with her partner's sperm, during ICSI.
Over 50 women underwent these interventions in Toronto, with some delivering babies. The health of these infants is unknown. In the absence of appropriate clinical trial evidence, the US Food and Drug Authority and the EU regulatory authorities denied the clinical use of this add-on, but Health Canada did not. Subsequently, a Spanish group presented and published the results of a randomised control trial of Augment vs no intervention and found that there was no benefit to the add-on (see BioNews 957).
A recent study from Monash University found that ICSI is used in 50 to 70 percent of IVF cycles worldwide, but severe male factor infertility only impacts 30 percent of couples. Publishing their results from a large, randomised trial in The Lancet, they confirmed that ISCI for non-severe male factor did not improve and may have significantly decreased the live birth rate while adding to the risk of congenital anomalies (see BioNews 1226).
The HFEA has determined that endometrial receptor assays reduce treatment effectiveness and time-lapse imaging and incubation have no effect on the treatment outcome. Where the UK and European guidelines clearly state the futility of specific add-ons, the Canadian recommendations are incomplete and couched in grey tones. Neither, the national fertility support organisation, Fertility Matters Canada, nor the CFAS has undertaken to inform the infertile about the futility of add-ons. As such the guidance document is not likely to be useful to patients who are desperate and willing to try anything, even if it doesn't improve the chance of a liveborn child. Canada does not have an independent fertility 'watchdog' to advise consumers.
More than 20 years ago, Dr John Collins, a Canadian academic obstetrician/gynaecologist and a respected global advocate for evidence-based medicine wrote that evidence-based medicine is the judicious and conscientious use of current best evidence from medical care research for making medical decisions. In his opinion evidence-based reproductive medicine does not replace sound clinical judgment, but it can provide access to the research evidence that is needed to implement good judgment.
Ineffective add-ons not only offer no benefit to patients and are very costly, but also carry potential negative implications for women, pregnancy, neonatal, and/or paediatric outcomes. We need to end our silence. Now is the time for Health Canada and its provincial/territorial partners in conjunction with interested parties to show leadership and to bring evidence-based guidelines to current reproductive medicine practice in Canada.
Leave a Reply
You must be logged in to post a comment.


Have Your Say
Yes, we need to end our silence.