Researchers have been developing human stem cell-based embryo models (SCBEMs) as a tool to study the early development of human embryos, and how errors in this process can cause developmental defects or recurrent miscarriages. There are important differences in the starting material, the stem cells, used for different SCBEM protocols, and this article will discuss the nuances of these human pluripotent stem cell (hPSC) states.
In the broadest terms, stem cells are defined by their dual capacity to self-renew and to mature into adult cell types. Some adult tissues, like the gut or circulating blood, contain 'adult stem cells', which can self-renew but can only generate a specific set of mature cells of their respective tissue. These are called 'multipotent' stem cells. Conversely, the zygote that is formed through fusion of sperm and egg is 'totipotent', as it readily generates the entire body and extra-embryonic tissues such as the placenta.
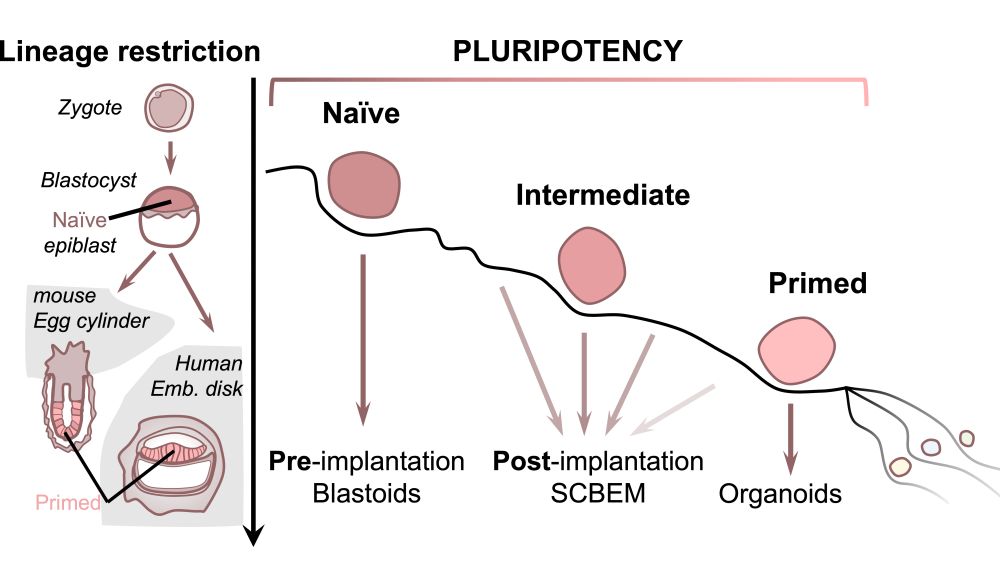
The 'pluripotent' stem cells used in SCBEMs arise at and following the formation of the blastocyst. Pluripotent cells emerge in the epiblast, the early inner structure of the blastocyst that makes the body. Mouse studies suggest that these cells transition through different 'states' of pluripotency. Cells in the epiblast start in a 'naïve' state, but upon implantation of the blastocyst into the maternal uterus, they transition to a 'primed' pluripotent state.
It is suggested that an intermediate state, sometimes referred to as 'formative', marks the point where the potential of the epiblast cells to generate the body is unlocked. However, translating findings in mice to the human context is complicated, not only due to low accessibility of human embryos for research, but also because of species-specific differences in pluripotency.
Pluripotency is a transient state during development, so capturing it in a dish has required tremendous effort. The protocols to generate pluripotent human embryonic stem cells (hESCs) from the blastocyst itself were reported in 1998, and were later complemented by a strategy to reprogram adult cells (such as skin cells) to produce induced pluripotent stem cells (iPSCs) in 2007.
In both cases, hPSCs are maintained in a 'primed' pluripotent state. In culture these cells tend to be flat, have a 'tiled' morphology, and rely on forming strong interconnections with one another. Primed hPSCs have proven useful for generating organoid models that contain specific mature cell types.
Previous work has also generated SCBEMs of the post-implantation embryo (the epiblast) and its derivatives, using primed cells. While primed hPSCs are useful for understanding development and disease, they only capture a late post-implantation stage of pluripotency (roughly 12-14 days post-fertilisation).
In the mid-2010s, several groups reported hPSCs that mirrored the earlier blastocyst-stage level of pluripotency. These naïve cells can be generated by converting primed hPSCs, or else can be derived directly from a primary blastocyst.
Equivalent mouse cells do not readily make extra-embryonic lineages, distinguishing these cells from mouse totipotent-like cells. However, mouse PSCs can be artificially induced to generate extra-embryonic lineages, when the PSCs are cultured in specific 'expanded' or 'extended' potential conditions.
While the terms 'expanded' and 'extended' have also been used to describe human conditions, this may be redundant, as it appears that non-primed human hPSCs can intrinsically generate extra-embryonic lineages in a dish. However, it remains unclear whether the human epiblast contributes to extra-embryonic cells in normal development.
Critically, the ability of naïve hPSCs to generate extra-embryonic cell types allowed several groups to create 3D blastocyst-like SCBEMs – called blastoids – from naïve hPSCs in 2021-2022. The resulting pre-implantation-like structures are impressive, yet remain unable to robustly develop towards a post-implantation-like state in a dish.
In recent months, new post-implantation-stage SCBEMs have been reported. Most of these do not rely on either fully naïve or fully primed cells. Instead, they harness the intermediate state of pluripotency. The relevant cells are still able to generate extra-embryonic cell types, particularly post-implantation stage yolk sac-like cells.
Naïve, intermediate, and primed starting hPSCs were tested in two of the recent reports, but only intermediate state cells could make those specific post-implantation structures. Interestingly, unlike blastoids, these SCBEMs never pass through a pre-implantation-like state.
Some recent research into post-implantation SCBEMs does use primed hPSCs as a starting point. Whether the extra-embryonic-like cells reported there are different from those generated with intermediate-state cells remains to be determined.
Although SCBEMs rely on our understanding of hPSCs and their capabilities, in the future, they – together with research using surplus human embryos – may serve as a tool to better understand pluripotency transitions. This knowledge will also improve the conditions used to culture hPSCs, and in turn the starting point of different SCBEMs moving forward.






Leave a Reply
You must be logged in to post a comment.